
Prof. John Hu
West Virginia University, USA
Title: Activation of Stable Chemical Bonds by Microwave Catalysis
Abstract:
Introduction
Microwave
catalytic processing is an emerging technology in line with the principles of
process intensification. The interaction of microwave with solid catalyst
through dielectric heating has been widely explored for numerous applications.1 Experimental evidence showed that microwave-induced activation process has
significantly lowered the activation energy of endothermic reactions, therefore
enabling the reaction to proceed under lower bulk temperature. Microwave-driven
catalytic process can quickly switch between startup and shutdown mode,
exhibiting flexibility in handling intermittent renewable power supply.
Microwave can also induce plasma to activate stable molecules. In the presence
of a catalyst, the pre-activated plasma species can react on the catalyst
surface under reaction pathways that are different from thermally heated
reaction. A synergy of the plasma species with catalyst can be established if
the post-plasma species influence the distribution and selectivity of the final
products. An optimum catalyst design can maximize the inter-molecular
interaction of the plasma species. This paper presents the applications of
microwave in CH4 conversion, NH3 synthesis and CO2 capture and utilization.
Materials and Methods
Microwave
testing was performed in a 2.45 GHz, 3 kW, magnetron generator with a mono-mode
cavity microwave from Sairem (model GMP20K). The temperature was measured using
a laser aligned infrared pyrometer (IR) from Micro-Epsilon (model CTLM-3SF75H2)
with a pre-calibrated temperature range between 200 and 1500 °C and a 6.5 mm
radial spot size. In the scenario of microwave plasma-driven catalysis, the CH4 and N2 are activated into plasma species in the microwave reactor
cavity. Plasma generation is performed using a 2.54 GHz, 3 kW, fixed frequency
microwave (Sairem, GMP20K). The quartz tube was placed in the waveguide at 300
W power in continuous wave mode, plasma was ignited using an external spark in
pure 50 sccm Ar (UHP, Matheson).
Results and Discussion
Brief
Experiments were carried out to form ethylene from ethane without using ZSM-5
catalyst. Figure 1 illustrates oxidative dehydrogenation of ethane using CO2 for ethylene production over CsRu based catalysts. The microwave reactor
reached 90% conversion at 650 °C, which is higher than that of a thermally
heated fixed-bed reactor operated at 800 °C. Ethylene yield is also higher
under the MW condition. Under microwave irradiation, the ratio of Ce3+/Ce4+ in CeO2 changes affecting the electron shift between Cs- Ru. This
could alter reaction pathways, leading to lower activation energy. The outcomes
of this work include decarbonizing the olefin industry and introducing
electrification into the process rather than simply changing the combustion
fired heater. The introduction of microwave heating to the process results in
“co-benefits” of improved energy efficiency and product yield.
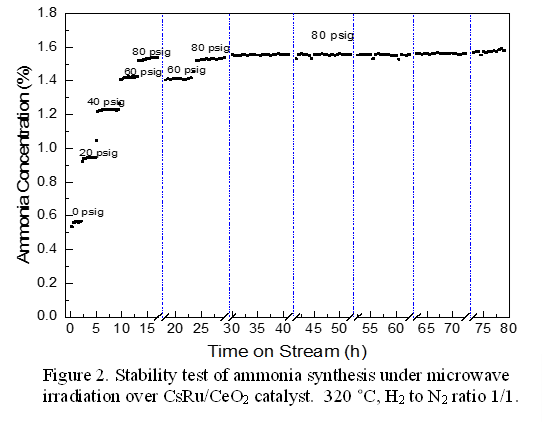
The effect of variation in pressure on
the performance of ammonia synthesis under microwave irradiation was
investigated. As shown in Figure 2, the stability test was performed
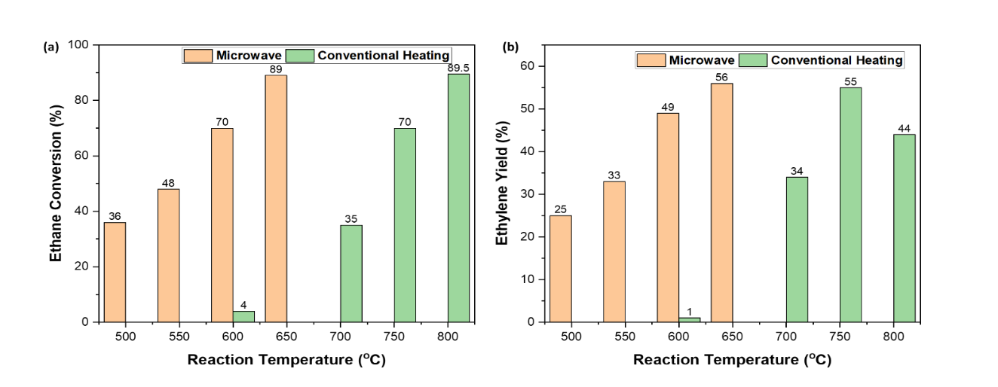
Figure 1. Oxidative
dehydrogenation of ethane by CO2 over Cs/Ru/CeO2 catalyst: comparison of microwave and conventional heating(a) conversion (b)
selectivity.
for 7 days, during which 6
cycles of startup- shutdown were performed.2 The total cumulative
on-line time was 80 hours. There was no loss in productivity either at 60 or 80
psig when microwave irradiation was resumed after shutdown. Five more cycles of
startup–shutdown was repeatedly performed at 80 psig, during the period of time
each cycle was held at 12 h on-line time. Ammonia concentration remained
constant during 6 cycles of repeated startup-shutdown operation. This
highlights that the microwave catalytic ammonia synthesis over the CsRu/CeO2 catalysts is a robust process with the flexibility of coping with intermittent
nature of renewable power supplies for distributed production.
Significance
Microwave-driven catalysis exhibits a
strong influence on the activation of stable molecules such as CH4,
ethane N2 and CO2. Microwaves improve energy intensity
and reduce carbon intensity, leading to decarbonization of chemical process via
electrification.
References
1. J.
Hu, Advances in Microwave-assisted Heterogeneous Catalysis, Book, RSC. 2023
2. Y. Wang, Catalysis
Communication, 2021, 159, 2021, 106344-.
Biography:
Dr. Jianli (John) Hu is a Chair Professor and the Director of Shale Gas Center at West Virginia University. He leads an interdisciplinary team carrying out cutting edge research in natural gas conversion and renewable energy utilization. He has demonstrated strong leadership in partnering with U.S. national laboratories and industrial companies. His research interests span across the fields of reaction engineering, surface chemistry, plasma and microwave-enhanced catalytic reactions. Before joining WVU, Dr. Hu led innovation efforts at Koch Industries, Pacific Northwest National Laboratory and BP Oil. He has been granted 45 U.S. patents and published over 120 peer-reviewed journal articles, and edited 2 books.