
Prof. Guangnan Ou
Jimei University, China
Title: Construction of artificial metalloenzymes by protein refolding
Abstract:
Metalloenzymes contain a metal cofactor in a protein scaffold,
which are involved in many biological processes such as nitrogen fixation,
respiration, hydrocarbon oxidation, and oxygenic photosynthesis. Based on the
function and mechanism of natural enzymes, in 2001, we proposed three catalytic
action modes that for the best one the negative charge centers seem to be the
active site for reactants or products, while the positive one seems to be the
active site for transition states, resulting in great decrease in the
activation energy. To meet the above requirements, artificial metalloenzymes
(ArMs) can be constructed by anchoring a metal complex of positive charge on
the pocket of a protein which periphery has negative charges.
To find a simple and versatile method for the incorporation of
metal complexes within the protein host, lessons can be learned from protein
folding. The protein folding forces include hydrophobic, electrostatic,
hydrogen bond, and van der Waals interactions. In the case of metalloproteins,
there is an additional folding force, ligand-metal interactions. It was
reported that there is a direct relationship between folding and metal
coordination. Inspired by metal-induced protein folding, we report here a
simple and versatile method for the incorporation of metal complexes within the
protein host by protein refolding,
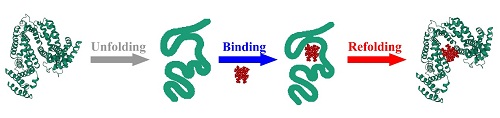
The as-prepared artificial hemeprotein was
characterized by UV-vis, CD, and MALDI-TOF-MS, which exhibited peroxidase-like
activities with an optical pH at pH 7.0 and an optical temperature at 65°C. The
Michaelis-Menten constant (Km) and maximum velocity (Vm)
with guaiacol for the as-prepared hemeprotein was calculated to be 0.131 mM and
0.166 mM/s, which was significantly lower compared with the value of 39.7 mM and
44.4 mM/s of native horseradish peroxidase. The artificial hemeprotein had much
more affinity to the guaiacol than HRP, and had lower guaiacol oxidation
efficiency than HRP.
Biography:
Guangnan Ou has completed his
PhD from Xiamen University. He worked as an Academic Visitor under the
supervision of Prof. Peter Halling at the University of Strathclyde for the
period 1 September 2013 to 31 August 2014. His research interests focus on
nonaqueous enzymatic catalysis, ionic liquid, chemical protein modification,
and artificial enzyme.