
Prof. Pogorelov Valeriy
Kyiv National Taras Shevchenko University, Ukraine
Title: Peculiarities of cluster structure and unique properties of supercooling water and monohydric alcohols
Abstract:
1. Intermolecular hydrogen bonding, which is formed
between water molecules, causes a variety of liquid water’ unique properties.
This manuscript presents the experimental FT IR and FT Raman studies' results
of water trapped in Ar matrix at temperatures from 10 to 50 K as well as
condensed water at temperatures from 100 to 370 K. It is shown that temperature
evolution for FTIR-spectra of water trapped in cryo Ar-matrices can be considered
as an experimental model of the water structure transformation during the phase
transition from gas phase to condensed confined water [1, 2]. The FTIR spectra
comparison of water in Ar-matrices with the corresponding spectra of bulk water
allows us to conclude that bulk water structure consists of clusters of
different sizes. These clusters are elementary volume units of hydrogen-bonded
networks. The comparison of the water vibrational spectra change with
temperature in Ar matrix and in condensed phase allows us to conclude that
intermolecular H-bonding can be seen in the water intracluster vibrational
spectra. They are absent in the isolated water molecules spectra.
2. At the measurements of FTRaman and FTIR spectra in
liquid phase (this and previous years) we can see shift broad OH-band with
increasing of temperature, that caused by valence vibrations (symmetrical and
unsymmetrical), to the high wavenumber. This could be explaining like this: in
a phase transition gas-liquid in case of intermolecular hydrogen bond appear
multymolecular associates (aggregates, clusters). These cause to such large,
abnormally (200-400sm-1) low frequency shift valence vibrations. Increase the
number of molecules in associates accompanied by low frequency shift,
broadening valence bands and increasing difference of frequencies between their
maximums. Then, the low frequency shift of frequency valence oscillations could
be linked with increasing associate sizes with decreasing of liquid water
temperature. These vibrational spectra contains information about peculiarities
of water cluster structure in cryomatrices and in condensed phase. Water
cluster structure in condensed phase is changing with temperature. Number of
large size clusters (4 – 6 molecules per cluster) is increasing while cooling
[3]. Thus water in condensed phase is a complex cluster structure, its’ cluster
composition is changing with temperature. Cluster compositions of different
sizes are depicting themselves through unusual water properties with unusual
behavior at different temperatures. Our investigation results allows us to
consider condensed water as a hydrogen bonded Continuous Molecular Networks,
where molecular clusters are structural units. One must take into account that
such networks are created from clusters as structural units, and for liquid
water the number of molecules in each cluster is not bigger than four. Networks
of supercooled water contains five-molecule clusters, what don’t allow to build
the crystalline structure and explain the water density decrease at
temperatures below 273 K (so-called low density water).
3. Based on the analysis of FTIR and FTRaman spectra,
the following conclusions can be drawn. When liquid water is cooled in the
range 0–-10°C, the number of five molecular clusters (pentamers) decreases,
while the number of six molecular clusters (hexamers) increases. With the
predominance of the content of hexamers in water, liquid water solidifies -
this is an intermediate transition of a liquid to a solid state. In the
temperature range 00C - the moment of crystallization, water is in a
supercooled state. When solid water (ice) is heated, the transition from solid
to liquid occurs at a temperature of 0°C without intermediate overcooling.
4. One can see the appearance in the Raman spectra
(RS) of a broad band (broadband background) in the liquid phase (Fig. 1 and
Fig. 2).
Previously, we
assumed that this phenomenon, recorded in the Raman spectrum of benzene and
some other liquids [4, 5], is associated with the appearance in the studied
liquids of submolecular quasi-linear structures that arise in liquids during
intermolecular interactions. So, in benzene, these can be structures where the
benzene rings are oriented parallel to each other. In such a substructure,
standing waves of electron density can arise The length of such waves is
determined by the sizes of these molecular substructures
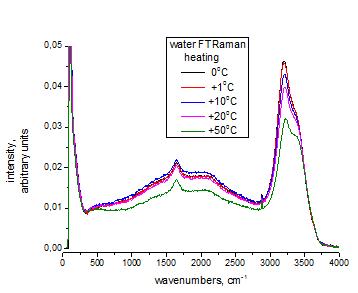
Fig.1. The background in FTRaman spectra liquid
water
electron density can arise. The length of such waves
is determined by the sizes of these molecular substructures that appear in the
Raman spectra as a broadband background. Knowing the thermal velocity of
electrons and the position of the background band maxima in the Raman spectra, it
is possible to calculate the linear dimensions of these substructures in a
light-scattering medium (water). The corresponding estimates I will add the
sizes and lifetimes of these electron formations. Here I mean the outer
electron shells of several neighboring clusters. These groups of clusters are
responsible for the appearance of a broadband background in the Raman spectra.
Naturally, such electronic formations appear only in the liquid phase and are
absent in the solid phase.
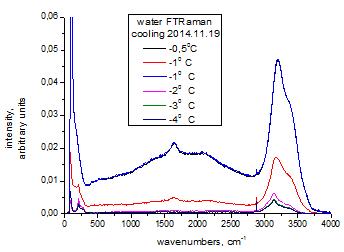
Fig.2. The broadband in FTRaman spectra
supercooled liquid and crystallic water
The length of such waves is determined by the
sizes of these molecular substructures that appear in the Raman spectra as a
broadband background. Knowing the thermal velocity of electrons and the
position of the background band maxima in the Raman spectra, it is possible to
calculate the linear dimensions of these substructures in a light-scattering
medium (water). The corresponding estimates I will add the sizes and lifetimes
of these electron formations. Here I mean the outer electron shells of several neighboring
clusters. These groups of clusters are responsible for the appearance of a
broadband background in the Raman spectra. Naturally, such electronic
formations appear only in the liquid phase and are absent in the solid phase.
In the presentation, The following conclusions can be
drawn based on the analysis of the FTIR and FTRaman spectra of water in the
temperature range +550 C - -500C, the following
conclusions are made:
1) When liquid
water is frozen in the range of 00C— -100C, the number of
five molecular clusters (pentamers) decreases and the number of six molecular
clusters (hexamers) increases. With a certain predominance of the hexamers’
concentration in water, liquid water solidifies - a liquid-solid phase
transition. In the temperature range of 00C - water
is in a supercooled state.
2) When solid water (ice) is heated, the solid-liquid
phase transition occurs at a temperature of 00C without intermediate
supercooling.
3) In the Raman spectra of water, molecular
polycluster substructures appear only in the liquid phase. Their number
decreases when liquid water is heated. These substructures caused the broadband
background in Raman spectra liquid water absent in the crystalline phase.
1. V. Pogorelov, I. Doroshenko, Low Temp. Phys. 42
(12) (2016) 1163 - 1166.
2. V. Pogorelov, I. Doroshenko, G. Pitsevich, V.
Balevicius, V. Sablinskas, B. Krivenko, L.G.M. Pettersson, From clusters to
condensed phase - FTIR studies of water, J. Mol. Liq. 235 (2017) 7 - 10.
3. A. Vasylieva, I. Doroshenko, Ye. Vaskivskyi, Ye.
Chernolevska, V. Pogorelov, FTIR study of condensed water structure, Journal of
Molecular Structure 1167 (2018) 232 – 238
4. J.P.Biscar and N.Kollias. (Chem. Phys. Lett., 1974,
V.26, 1, p.82 – 84 and Chem. Phys., Lett., 1974, V.27, 1, p.100 – 102.
5. V. Pogorelov, G.Salivon, I.Klassen Ukr. Phys.
Journ, 1987, v.32, no. 9, p.1342-1345.
6, V.Pogorelov, I.Doroshenko Trends in Physical Chemistry, 2023, Volume
23, p. 35-40.
Biography:
Prof. Pogorelov Valeriy studied Physic (Molecular Spectroscopy) at the Kyiv National Taras Shevchenko University, Ukraine and graduated as PhD in 1966. He obtained the position Full Professor at same University in 1986. His scientific interests are Raman Spectroscopy, vibrational and orientational molecular relaxation, structure and spectroscopy partially ordered liquids, peculiarities of cluster structure of water and 10 first alcohols. He has published 6 books (last book is “Cluster structure of water”, V.Pogorelov, I.Doroshenko, A.Vasiiieva, “Lambert”, 146 p., 2022) and more than 200 research articles in SCI(E) journals.